The chemistry and biological activity of the Hyacinthaceae
Abstract
Covering: 1914 to 2012
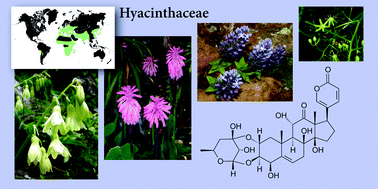
The Hyacinthaceae (sensu APGII), with approximately 900 species in about 70 genera, can be divided into three main subfamilies, the Hyacinthoideae, the Urgineoideae and the Ornithogaloideae, with a small fourth subfamily the Oziroëoideae, restricted to South America. The plants included in this family have long been used in traditional medicine for a wide range of medicinal applications. This, together with some significant toxicity to livestock has led to the chemical composition of many of the species being investigated. The compounds found are, for the most part, subfamily-restricted, with homoisoflavanones and spirocyclic nortriterpenoids characterising the Hyacinthoideae, bufadienolides characterising the Urgineoideae, and

 Please wait while we load your content...
Please wait while we load your content...