Heme to protein linkages in mammalian peroxidases: impact on spectroscopic, redox and catalytic properties†
Abstract
Covering: 1966 to 2007
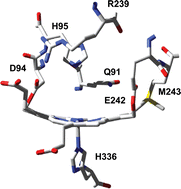
The mammalian peroxidases myeloperoxidase, eosinophil peroxidase, lactoperoxidase and thyroid peroxidase participate in host defense against infection, hormone synthesis and pathogenesis. The most striking feature of these heme peroxidases is the existence of two covalent ester bonds between the prosthetic group and the protein in the functional, mature enzymes. Myeloperoxidase (MPO) is unique in having an additional vinyl–sulfonium bond. This review presents our knowledge about the mechanisms for the covalent bond formation and its role in protecting the heme of these peroxidases from modification by their own reaction products. The impact of heme distortion and asymmetry on the spectral and enzymatic properties is discussed as is the role of the MPO-typical electron withdrawing sulfonium ion linkage in raising the reduction potential of its redox intermediates and maintaining a rigid solvent network at the distal heme cavity. These structural features allow MPO to be the only human enzyme that efficiently binds and oxidizes chloride to antimicrobial hypochlorous acid.
- This article is part of the themed collection: Heme

 Please wait while we load your content...
Please wait while we load your content...