Insights into the existing form of glycolaldehyde in methanol solution: an experimental and theoretical investigation†
Abstract
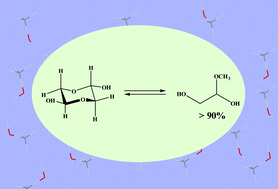
Glycolaldehyde (HOCH2CHO, GA), the simplest molecule containing both hydroxyl and aldehyde groups, is structurally the most elementary member of monosaccharide sugars, which may provide new clues for probing the origin of life on planets like the Earth. Uncovering the existing state of GA in solution systems is an important scientific issue. Generally, methanol is used as the main mobile phase in the liquid chromatography analysis of GA, but the state of GA existing in methanol solution remains unknown, thus making it difficult to analyse GA accurately. Herein, the state and dynamic equilibrium of GA in methanol solution were systematically studied by UV-visible spectroscopy, nuclear magnetic resonance (1H-NMR) spectroscopy, liquid chromatography mass spectrometry (LC–MS) and density functional theory (DFT) calculations. The results demonstrated that the equilibrium component of GA in methanol solution is different from that in aqueous solution and that glycolaldehyde hemiacetal (GAHA) is a dominant component (>90%). Laying the foundation for experimental analysis, the transformation of different components at equilibrium was studied using DFT. The results confirm that hydrogen bonding-induced proton transfer occurs between the components at equilibrium. This work provides an important reference for the analysis of sugars and related compounds in various biochemical reactions.


 Please wait while we load your content...
Please wait while we load your content...