Rhodium(i) N-heterocyclic carbene complexes: synthesis and cytotoxic properties†
Abstract
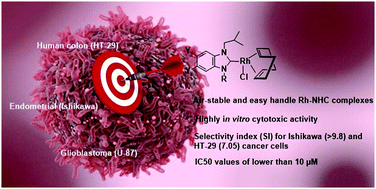
Rhodium(I) complexes bearing N-heterocyclic carbene (NHC) ligands have been widely used in catalytic chemistry, but there are very few reports of biological properties of these types of complexes. A series of benzimidazolium salts and their [RhCl(NHC)(COD)] complexes were synthesized. The obtained complexes were synthesized and characterized by elemental analysis, FT-IR, 1H and 13C NMR. All compounds were screened for in vitro cytotoxic activities against a panel of human cancer cells (HT-29 colon, Ishikawa endometrial, and U-87 glioblastoma) using the MTT assay for 48 h of incubation time. Mouse fibroblast cells (L-929) were used as healthy cells. Complexes had exhibited significantly higher cytotoxic activity towards cancer cells than their ligands and complex 2b showed the most selective cytotoxic activity against HT-29 cancer cells (SI;7.05) and Ishikawa cancer cells (SI; more than 9.8). The complexes showed strong in vitro cytotoxic activity against cancer cells, with IC50 values of lower than 10 μM (except 2a against HT-29 (12.8 μM) and 2b against U-87 (11.1 μM)). All complexes (2a–d) showed the highest in vitro cytotoxic activity against Ishikawa endometrial cancer cells with IC50 values of 2.93 ± 0.06, <1, 2.60 ± 0.05, and 2.85 ± 0.06 μM, respectively. Complexes were found to be highly cytotoxic against HT-29, Ishikawa, and U-87 cancer cells compared to the anticancer agents, cisplatin and 5-FU.


 Please wait while we load your content...
Please wait while we load your content...