Stereoselectivity in dehydrative cyclic trimerization of substituted 4-alkylaminobenzoic acids†
Abstract
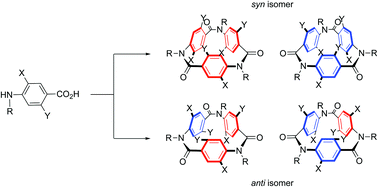
The cyclic trimerization of substituted 4-alkylaminobenzoic acids was investigated. From NMR analyses of DiMeO_C3A with two methoxy groups, which was obtained by using SiCl4 as a dehydrative condensation reagent and purified by preparative GPC, a syn/anti ratio of 60/40 was obtained. On the other hand, 3Br_C3A with one bromine group at the ortho-position relative to the amide nitrogen was synthesized by using PPh3/Cl3CCCl3 as a dehydrative condensation reagent and isolated by SiO2 column chromatography. 3Br_C3A showed an inverse stereoselectivity, namely, a syn/anti ratio of 25/75 was calculated based on the comprehensive NMR analyses. The population of stereoisomers had no relationship with the dehydrative condensation reagent and reaction temperature. The solvent character also had a negligible influence on the syn/anti ratio in solution reflecting the rigid structure of 3Br_C3A.


 Please wait while we load your content...
Please wait while we load your content...