Chalcogen bonding interactions in chelating, chiral bis(selenocyanates)†
Abstract
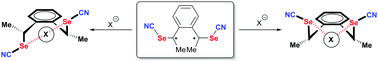
Introduction of methyl substituents on the achiral 1,2-bis(selenocyanatomethyl)benzene leads to a novel chelating ChB donor, namely 1,2-bis(1-selenocyanatoethyl)benzene (1), as a mixture of three diastereomers, the two anti enantiomers and the syn (meso) form. Structure determinations show the recurrent formation of short Se⋯N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C ChB interactions in both the anti (racemic mixture) and syn isomers. Co-crystallization of anti-1 with 4,4′-bipyridine affords a 2 : 1 adduct, (anti-1)2(bipy), with one very short Se⋯NPy ChB (RR = 0.87). Co-crystallization of anti-1 with tetraphenylphosphonium halides (Cl−, Br−, I−) provides 1 : 1 adducts while a 2 : 1 adduct is isolated between syn-1 and Et4NCl, formulated as Et4N+[(syn-1)2Cl−]. Comparison of chloride chelation with anti-1 and syn-1 shows much shorter (NC)Se⋯Cl− ChB interactions with the syn isomer, tentatively rationalized on the basis of theoretical calculations of (i) the electrostatic surface potential of neutral ChB donors and (ii) the chloride BSSE complexation energy.
C ChB interactions in both the anti (racemic mixture) and syn isomers. Co-crystallization of anti-1 with 4,4′-bipyridine affords a 2 : 1 adduct, (anti-1)2(bipy), with one very short Se⋯NPy ChB (RR = 0.87). Co-crystallization of anti-1 with tetraphenylphosphonium halides (Cl−, Br−, I−) provides 1 : 1 adducts while a 2 : 1 adduct is isolated between syn-1 and Et4NCl, formulated as Et4N+[(syn-1)2Cl−]. Comparison of chloride chelation with anti-1 and syn-1 shows much shorter (NC)Se⋯Cl− ChB interactions with the syn isomer, tentatively rationalized on the basis of theoretical calculations of (i) the electrostatic surface potential of neutral ChB donors and (ii) the chloride BSSE complexation energy.


 Please wait while we load your content...
Please wait while we load your content...