Ligand effects on structural, protophilic and reductive features of stannylated dinuclear iron dithiolato complexes†
Abstract
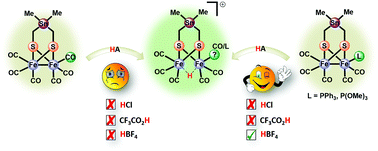
The synthesis and characterization of Fe2(CO)5(L){μ-(SCH2)2SnMe2} (L = PPh3 (2) and P(OMe)3 (3)) derived from the parent hexacarbonyl complex Fe2(CO)6{μ-(SCH2)2}SnMe2 (1) are reported. Whereas 1 exhibits a unique planar structure, X-ray crystallography showed that the apical orientation of L in complexes 2 and 3 results in a chair/boat conformation of the Fe2S2C2Sn fused six-membered rings, which is typical for diiron dithiolato complexes. In solution, NMR and FTIR spectroscopic techniques provide evidence for a dynamic process of apical–basal site exchange of the ligand L in 2 and 3. Protonation experiments on 2 and 3 in MeCN using CF3CO2H, HCl or HBF4·Et2O suggest enhanced protophilicity of the Fe–Fe bond due to the presence of the electron donor ligands L as well as the stannylation effect. While the carbonyl ligands in 2 stretch at lower wavenumbers ν(CO) than those in 3, the cyclic voltammetric reduction of 2 unpredictably occurs at less negative potential than that of 3. In contrast to 1, the presence of PPh3 and P(OMe)3 in 2 and 3, respectively, allows protonation prior to reduction as shown by FTIR spectroscopy and cyclic voltammetry.


 Please wait while we load your content...
Please wait while we load your content...