Aza-heterocyclic frameworks through intramolecular π-system trapping of spiro-N-acyliminiums generated from isoindolinone†
Abstract
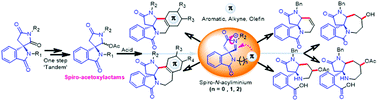
Spiro-acetoxylactams as key substrates were obtained straightforwardly by the tandem regioselective reduction/O-acylation of spiro-isoindolinone-imides. The latter were produced in large scale in three steps from homophthalic acid. These imides were useful to provide in one step isoindolinones bearing hydroxymethyl-amide functions, tethered at quaternary carbon centre with promising biological issues. Submitted to Brønsted (TFA neat) and Lewis acid (trimethylsilyltrifluoro-methanesulfonic (TMSOTf) 10 mol%), the spiro-acetoxylactams undergo π-cyclization of spiro-N-acyliminiums to provide diastereoselectively with π-aromatic original pyrrolopyrido- and pyrroloazepino-isoindoles fused or not to aromatic systems. With π-olefins, pyrrolopyridines and pyrrolo-azepines fused to isoindoline or containing amino-acids were isolated. A reaction mechanism producing all these systems is also discussed.


 Please wait while we load your content...
Please wait while we load your content...