One-pot asymmetric synthesis of a hexahydrophenanthridine scaffold containing five stereocenters via an organocatalytic quadruple-cascade reaction†
Abstract
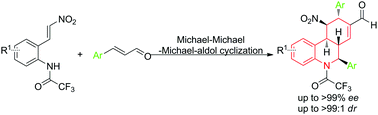
An organocatalytic enantioselective aza-Michael-Michael-Michael/aldol cyclization quadruple-cascade reaction of 2-amino-β-nitrostyrenes and α,β-unsaturated aldehydes has been developed for the construction of fully substituted hexahydrophenanthridine. This cascade reaction was efficiently catalyzed by diphenylprolinol TMS ether furnishing the tricyclic compounds containing hexahydrophenanthridine bearing five contiguous stereogenic centers with excellent diastereoselectivities (up to >99 : 1 dr) and high to excellent enantioselectivities (up to >99% ee). Application in the gram-scale synthesis of the tricyclic compounds containing hexahydrophenanthridine was also successfully realized.


 Please wait while we load your content...
Please wait while we load your content...