A novel colorimetric and ratiometric fluoride ion sensor derived from gallic acid†
Abstract
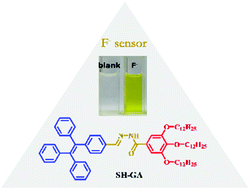
A novel colorimetric and ratiometric probe (SH-GA) for the fluoride ion detection was designed and synthesized from gallic acid. SH-GA exhibited a selective color change from colorless to yellow in the presence of F− among the eight anions (F−, Cl−, Br−, I−, H2PO4−, HSO4−, CH3COO−, and ClO4−), either in THF or DMF solutions. The binding stoichiometry between SH-GA and fluoride ions was determined to be 1 : 1, and the complexation constant was 1.5 × 103 M−1 in THF. The detection limit of the SH-GA probe reached as low as around 1 μM in THF. 1H NMR titration results and DFT calculations suggested a deprotonation process via hydrogen bonding interactions between the fluoride ion and amino group of SH-GA.


 Please wait while we load your content...
Please wait while we load your content...