Stimulus-responsive conformational transformation of peptide with cell penetrating motif for triggered cytotoxicity†
Abstract
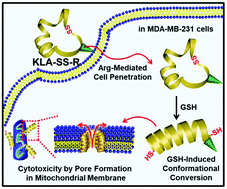
The KLA peptide, an antimicrobial peptide with an α-helical structure, needs to improve its low therapeutic efficacy and specificity for utilization as an anticancer agent. Here, we developed a modified KLA peptide (KLA-SS-R) with an intramolecular disulfide bond and a cell penetrating sequence for enhanced endocytosis and triggered cytotoxicity towards cancer cells. The intramolecular disulfide bond of KLA-SS-R sufficiently suppresses not only the helicity but also the cytotoxicity in the absence of a reducing agent. Upon treatment of KLA-SS-R to the cancer cells, the cell penetrating sequence contributes to enhanced intracellular uptake. Then, the cytoplasmic reducing agent secreted by cancer cells reduces the intramolecular disulfide bond of KLA-SS-R. Consequently, KLA-SS-R exhibits stimulus-responsive conformational transformation from low to high helicity and thereby selective cytotoxicity towards cancer cells by disruption of the plasma and mitochondrial membranes.


 Please wait while we load your content...
Please wait while we load your content...