Structure, photoluminescence, and magnetic properties of a Mn(ii)-based metal–organic framework†
Abstract
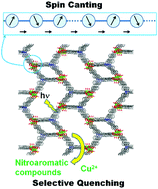
We synthesized a three-dimensional Mn-based framework [Mn3L2(H2O)3]·7.5DEF·3H2O (1; H3L = tris((4-carboxyl)phenyl)amine, DEF = N,N-diethylformamide) via the solvothermal reaction of Mn2+ and H3L in a mixed DEF/H2O solvent. In the crystal structure of 1, two types of 4- and 8-membered ring channels in the lattice emerge. Three Mn centers in the asymmetric unit adopted distorted octahedral (Mn2 and Mn3) and trigonal bipyramidal (Mn1) arrangements. The photoluminescence of 1 was completely quenched by nitroaromatic compounds and Cu2+ ions. Magnetic properties were studied as a function of temperature and magnetic field, and the results indicated the existence of overall antiferromagnetic couplings between the Mn(II) spins. Weak ferromagnetism was observed at low temperatures due to the possible spin canting in this Mn(II) system.


 Please wait while we load your content...
Please wait while we load your content...