Fenton-like removal of tetracycline from aqueous solution using iron-containing carbon dot nanocatalysts
Abstract
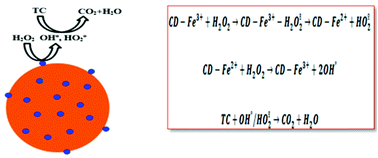
Nowadays the use of large-scale agro and forest-based waste is of worldwide research interest to provide composite nanocatalysts for wastewater treatment applications. In this work, a forest-based local feedstock as a natural resource was applied for synthesizing environment-friendly carbon dots. Fe-containing CD nanocatalysts were prepared using a simple one-pot hydrothermal route (180 °C for 2 hours) and used for tetracycline removal by a Fenton-like reaction. Experiments were designed by response surface methodology and the effects of the hydrogen peroxide amount (2.5–7.5 mM), catalyst dose (100–500 mg l−1), pH (3–11), and time (5–50 min) on the removal efficiency were studied using a Box–Behnken design. The catalyst was first characterized by various experimental techniques of SEM, TEM, XRD, and FTIR. The as-synthesized Fe-containing CD nanocatalysts have an average size of 50 nm. The nanocatalyst composite contains carbon, nitrogen, oxygen, and iron with weight percentages of 22, 3, 56, and 19% respectively. The Fenton-like reaction catalysed by the new nanocatalyst was able to remove more than 95% of 50 mg l−1 tetracycline in just 37 minutes. Experiments show that the contribution of adsorption was negligible and catalytic oxidation by hydroxyl radicals is responsible for the fast pollutant removal. The nanocatalyst shows good stability and preserves its activity for 5 successive runs.


 Please wait while we load your content...
Please wait while we load your content...