Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: experimental and computational investigations†
Abstract
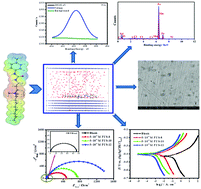
The facile synthesis approach, surface-active properties, and anti-corrosive efficiency of innovative urea-based cationic fluorinated surfactants (FUS) of variable alkyl chain length were investigated. The effect of as-prepared FUS inhibitors on carbon steel corrosion in 15% HCl solution at 20–50 °C was examined using weight loss and various electrochemical methods (open circuit potential (OCP) vs. time, potentiodynamic polarization (PDA) and electrochemical impedance spectroscopy (EIS) methods). The corrosion protection capacities at an optimum concentration (0.5 mM) are 93.2% (FUS-8), 96.3% (FUS-10) and 97.6% (FUS-12) by PDA, comparable to the findings achieved by EIS. PDA measurements indicated that the studied FUS act as inhibitors of mixed-type and chemical adsorption on a carbon steel interface following the Langmuir isotherm model. The thermodynamic and kinetic indices of the corrosion and adsorption processes were estimated and interpreted. Surface examinations by X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FTIR), field emission scanning electron microscopy and energy dispersive X-ray analysis (FESEM/EDX) revealed the formation of a protecting layer at the carbon steel/HCl interface. Quantum chemical indices (DFT based) delivered more understanding of the inhibition mechanism. Molecular dynamics (MD) simulations were accomplished to explore the configurational adsorption performance of the studied fluorosurfactants on the iron(110) interface. It is supposed that these findings are of some significance for the reasonable design of effective inhibitors for acidic corrosion.


 Please wait while we load your content...
Please wait while we load your content...