Boosting glucose oxidation by constructing Cu–Cu2O heterostructures†
Abstract
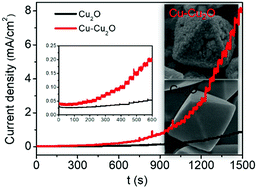
Cuprous oxide (Cu2O) is an important p-type semiconductor and widely used in the electrocatalytic field. However, the limited number of active sites and its poor conductivity make it difficult to further improve its catalytic activity. Here, we adopt a two-step method involving the reduction of Cu2+ at alkaline conditions and low temperature as well as heat treatment to fabricate Cu–Cu2O heterostructures in the presence of PVP. When used as a catalyst for glucose oxidation, Cu–Cu2O heterostructure possesses abundant active sites to react with glucose molecules, and moreover the metallic Cu simultaneously provides a highly conductive path for electrical charge transfer during the electrocatalytic oxidation reaction of glucose. As a result, the Cu–Cu2O heterostructure exhibits enhanced electrocatalytic activity towards glucose oxidation in 0.1 M NaOH solution compared to only Cu2O, and the fabricated non-enzymatic glucose sensor offers a low detection limit of 1.4 μM (S/N = 3), a high sensitivity (1446 μA mM−1 cm−2), and a wide linear range (0.002–4 mM) at a working voltage of 0.55 V (vs. SCE) in alkaline solutions. The as-prepared Cu–Cu2O heterostructure has a high catalytic activity, providing a new strategy for the design of other electrocatalysts.


 Please wait while we load your content...
Please wait while we load your content...