On the recognition of chloride, bromide and nitrate anions by anthracene–squaramide conjugated compounds: a computational perspective†
Abstract
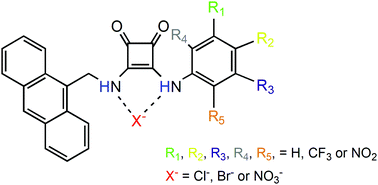
Anion recognition is widely used in several biological fields. Squaramide derived compounds appear as potential structures to recognize anions. Here, the bond mechanisms between the chloride (Cl−), bromide (Br−) and nitrate (NO3−) anions and anthracene–squaramide conjugated compounds are elucidated considering the influence of the: (i) number, (ii) nature, and (iii) position of the substituents: trifluoromethyl (–CF3) and nitro (–NO2). Energy decomposition analysis (EDA) shows that the interactions between Cl−, Br− and NO3− and anthracene–squaramide have an attractive interaction energy supported predominantly by electrostatic energy followed by orbital contribution. Molecular electrostatic potential (MEP) surfaces imply electrostatic interactions between Cl−, Br− and the oxygen atom from NO3− and the hydrogen atoms from N–H and C–H bonds present in the squaramide structure, and an aryl group, respectively. Cl− interacts with the receptors more strongly than Br−. The NO3− recognition is less attractive than those presented by Cl− and Br−, in agreement with the hardness–softness features of these anions. Importantly, one and, mostly, two group substitutions, –H → –CF3 or –NO2, favor the recognition of Cl−, Br− and NO3− due to the increase of the polarization in the receptor–NH⋯anion interaction. The –NO2 group promotes a larger effect relative to the –CF3 ligand. The –NO2 ligand positioned at the largest distance conceivable to the benzene–NH group promotes the lowest interference in the N–H⋯Cl− interaction. These results provide information to design receptors with a larger capability to recognize anions.


 Please wait while we load your content...
Please wait while we load your content...