Tetra- and poly-nuclear Cd(ii) complexes of an N3O4 Schiff base ligand: crystal structures, electrical conductivity and photoswitching properties†
Abstract
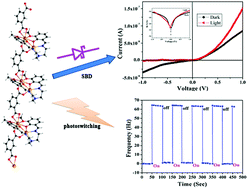
Two new Cd(II) complexes, [Cd4L2(NO3)2(μ1,1-N3)2(CH3OH)2] (1) and {[Cd2L(IPA)]2·(CH3OH)}n (2), have been synthesized by using a multidentate Schiff base ligand H2L (N,N′-bis(3-methoxysalicylidene)-diethylenetriamine) and two bridging co-ligands azide and isophthalic acid (IPA), respectively. Single crystal structure analysis shows that complex 1 has a tetranuclear structure whereas complex 2 forms a 1D polymer. The photoswitching behavior of both complexes has been measured under 1 Sun illumination. A significant enhancement of frequency ∼65 Hz was observed for complex 2, but for complex 1 no such enhancement was detected. To gain a detailed understanding about the charge transport mechanism of the complexes, the effective carrier mobility, transit time, carrier concentration and diffusion length have been estimated considering Schottky Barrier Diode (SBD) characteristics. The charge transport properties of complex 2 were found to be superior to complex 1. Complex 2 showed increased conductivity from 5.4 × 10−8 S cm−1 to 135 × 10−8 S cm−1 in the presence of light.


 Please wait while we load your content...
Please wait while we load your content...