Structure of the flavonoid catechin in solution: NMR and quantum chemical investigations†
Abstract
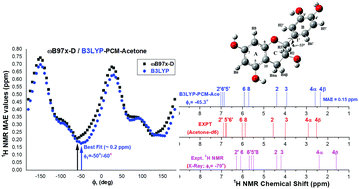
The knowledge of the molecular structure in solution is important for further molecular modeling studies on the drug interaction with biological targets. In this work we investigated the preferred molecular structure of the flavonoid catechin in acetone solution and water using density functional theory (DFT) methods. The performance of the quantum chemical methods for predicting experimental NMR spectra was assessed using nine DFT functionals along with the ab initio post-Hartree–Fock method as well as standard double-zeta and triple-zeta quality basis sets. The B3LYP/6-31G(d,p)-PCM level of calculation displayed the best agreement with NMR experimental data at a reasonable computational cost. The conformation of catechin in solution was assessed by calculating NMR chemical shifts through the potential energy surface (PES) varying the inter-ring dihedral angle and the position of the hydroxyl side-groups. In this strategy, the predicted chemical shifts are compared to the reference value and the global and some local minima are candidates as the observed molecular structure in solution. The results suggest that the catechin molecular conformation present in solution deviates significantly from that in the solid state, which is a structural information commonly used in studies of drug interactions with biological targets, such as DNA. Moreover, the conformation of the five OH groups present in the catechin molecule in solution was determined, which is relevant information and hard to obtain by only analyzing experimental NMR data.


 Please wait while we load your content...
Please wait while we load your content...