Glycosylation with ulosonates under Mitsunobu conditions: scope and limitations†
Abstract
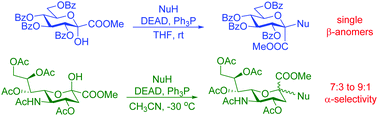
A systematic study was performed by using Mitsunobu conditions (diethyl azodicarboxylate, Ph3P or n-Bu3P in THF or CH3CN) for glycosylations with methyl 3,4,5,7-tetra-O-benzoyl-α-D-gluco-hept-2-ulopyranosonate. From a set of 47 O-, N-, S- and C-nucleophiles, phenols and N-hydroxy compounds with a pKa of 5–8, phthalimide, benzotriazole, 6-chloropurine, an oxazolidinedione and several tetrazoles with a pKa of 4–8, and thiophenol gave the corresponding products in moderate to very good yields, while C-nucleophiles were unreactive. Trihaloacetanilides underwent O-glycosylation to give O-glycosyl-N-aryl trihaloacetimidates which could not be made by conventional O-imidoylations. All reactions were highly stereoselective to produce the β(D) anomers only. With methyl (5-acetamido-4,7,8,9-tetra-O-acetyl-3,5-dideoxy-D-glycero-D-galacto-2-nonulopyranose)onate phenols and benzotriazole resulted in the expected products, but all other nucleophiles failed to react. While these transformations gave anomeric mixtures in a ratio close to 1 : 1 at room temperature, the α-selectivity increased to 92 : 8 at −30 °C. An o-nitrophenyl sialoside was converted to a spiro-benzoxazinone derivative by reduction of the nitro group and subsequent spontaneous ring closure.


 Please wait while we load your content...
Please wait while we load your content...