A new fluorescent hemicryptophane for acetylcholine recognition with an unusual recognition mode†
Abstract
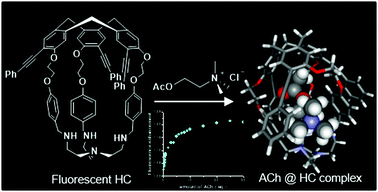
A new off–on fluorescent hemicryptophane probe for acetylcholine has been designed. This hemicryptophane, made fluorescent via an extension of the conjugation of its cyclotriveratrylene C3-symmetry part, exhibits improved fluorescence properties compared with fluorescent hemicryptophanes previously described. Indeed, both the excitation and emission wavelengths are red-shifted and the quantum yield is increased. Moreover, this hemicryptophane is able to bind acetylcholine with a high association constant of 3.2 × 104 M−1. This recognition process is accompanied by an increase in the brightness of the capsule. Surprisingly, contrary to what is commonly observed with cyclotriveratrylene-based hosts, the quaternary ammonium of the guest interacts with the tris(2-aminoethyl)amine south part of the hemicryptophane instead of the cyclotriveratrylene north part. This unusual binding mode is supported by both proton NMR experiments and density functional theory calculations.


 Please wait while we load your content...
Please wait while we load your content...