Pyrazolylphenanthroimidazole heterocycles: synthesis, biological and molecular docking studies†
Abstract
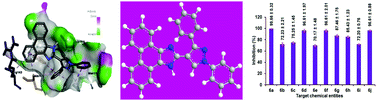
The synthesis of a series of novel pyrazolylphenanthroimidazoles 6a–6j has been accomplished utilizing a multi-step synthetic protocol, and characterized through physical and spectral techniques. Among them, the molecules possessing para-bromo (6d), para-methyl (6f) and para-nitro (6j) phenyl substituents on the pyrazole scaffold displayed similar anti-inflammatory activity and the one with no substituents on the aryl unit (6a) exhibited the highest anti-inflammatory profile. While investigating the DPPH radical scavenging activity, the synthesized chemical entity with a para-methoxyphenyl group attached at the pyrazole structural motif (6i) revealed the highest activity when compared to the other synthesized molecules. Furthermore, the evaluation of cytotoxic activity of the synthesized molecule (6a) exerted significant activity against both the pancreatic cell lines such as AsPC1 and SW1990. Besides, while performing the molecular docking studies of 6a with B-cell lymphoma 2, an appreciable binding affinity (−9.04 kcal mol−1) has been observed. The results of the present examination imply that these chemical entities could be used as efficient intermediates for the construction of biopertinent molecules.


 Please wait while we load your content...
Please wait while we load your content...