Synthesis of new fluorescent pyrylium dyes and study of their interaction with N-protected amino acids†
Abstract
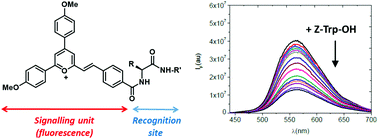
Six new 6-styryl-2,4-diarylpyrylium salts have been synthesized and fully characterized by means of 1H/13C NMR, HRMS, UV-vis and Steady-State/Time-Resolved Fluorescence spectroscopies. This set of molecules is composed of a core pyrylium fluorophore, an amino acid (valine or phenylalanine), and an alkylic chain of variable length. The emissive properties (fluorescence quantum yields and lifetimes and radiative deactivation rate constants) in dichloromethane, acetonitrile and PBS have been recorded. The interaction of these pyrylium salts with aminoacids in their N-protected forms has been studied by means of fluorescence quenching, using the Stern–Volmer methodology. It has been found that dynamic (collisional) quenching is the most prevalent process for all the fluorescent molecules, irrespective of the amino acid building block or the length of the alkyl chain. The emission of the pyrylium molecules is strongly quenched by Z-Trp-OH and to a lesser extent by Z-Tyr-OH and Z-Met-OH and no quenching was measured with Z-Ala-OH, Z-Val-OH and Z-Phe-OH. A small degree of ground-state complexation was observed for receptor 8a and by Z-Trp-OH (upward curvature in the Stern–Volmer plot). Complementary 1H-NMR titrations demonstrated the existence of such a weak ground state complex.


 Please wait while we load your content...
Please wait while we load your content...