Metal free highly efficient C–N bond formation through 1,6-addition: synthesis and photophysical studies of diaryl methyl amino acid esters (DMAAEs)†
Abstract
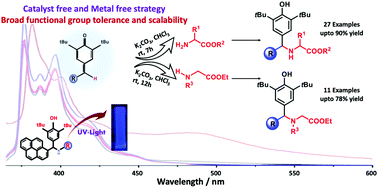
A transition-metal free, proficient strategy for the one-pot synthesis of diverse diaryl methyl amino acid esters (DMAAEs) has been established from the easily accessible chiral amino acid esters and para-quinone methides (QMs) in very good to excellent yields. Interestingly, peptide molecules having multifunctionalities also reacted chemoselectively with p-QMs in very good yield. The photophysical properties such as emission wavelength and fluorescence efficiency of DMAAEs are significantly dependent on the solvent polarity/nature. A confocal Raman spectroscopy study indicates the structural similarities leading to similar photophysical responses of pyrene derivatives.


 Please wait while we load your content...
Please wait while we load your content...