A novel carbazolyl GFP chromophore analogue: synthesis strategy and acidic pH-activatable lysosomal probe for tracing endogenous viscosity changes†
Abstract
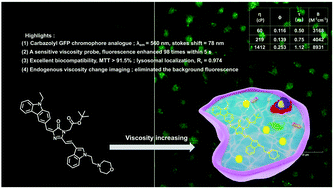
Intracellular viscosity changes of the internal microenvironment lead to many diseases, including cancer, inflammation, and neurodegenerative diseases. A novel, acidic pH-activatable carbazolyl GFP chromophore analogue, Lys-CzFP, was designed for tracing lysosomal viscosity changes. The developed synthesis is an efficient, novel, one-pot procedure. Lys-CzFP serves as a fluorescent molecular rotor and the internal carbazole and benzazole moieties of Lys-CzFP serve as rotators, which can rotate around the single C–C bond in the π-conjugated bridge. Lys-CzFP showed a long emission wavelength at 560 nm and a large Stokes shift of 78 nm. The fluorescence intensity of Lys-CzFP exhibited a significant enhancement with increasing viscosity. The fluorescence intensity increased by 98-fold within 5 s from 1.0 cP to 1412 cP, while the fluorescence quantum yield (ϕF) increased from 0.003 to 0.253 and the fluorescence lifetime increased from 0.25 ns to 1.12 ns, respectively. Furthermore, interference experiments indicated that Lys-CzFP could particularly respond to viscosity. Additionally, Lys-CzFP had excellent biocompatibility, low cytotoxicity, and lysosomal localization. Finally, Lys-CzFP was successfully applied to tracing endogenous viscosity changes in living cells.


 Please wait while we load your content...
Please wait while we load your content...