Effect of a mixed precursor over monolith MnOx/La–Al2O3 catalyst for toluene oxidation
Abstract
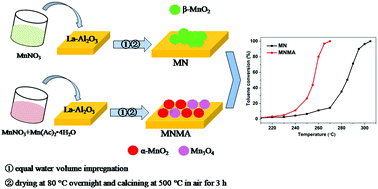
Monolithic MnOx/La–Al2O3 catalysts were prepared using manganese nitrate, manganese acetate and their mixed precursor for the catalytic oxidation of toluene. The activity results indicated that the MNMA catalyst prepared with the mixed precursor exhibited the best catalytic activity and that complete conversion of toluene was achieved at 270 °C under a GHSV of 12 000 h−1, which was 35 °C lower than the conversion of the MN catalyst prepared by manganese nitrate. N2 adsorption–desorption, X-ray diffraction (XRD), Raman spectroscopy, high resolution trans-mission electron microscopy (HRTEM), H2 temperature-programmed reduction (H2-TPR), O2 temperature-programmed desorption (O2-TPD), X-ray photoelectron spectroscopy (XPS) and in situ infrared diffuse reflectance spectroscopy (in situ DRIFTS) techniques were employed for the characterization of the catalysts’ properties. It was shown that the MNMA catalyst has better MnOx dispersion and more active α-MnO2 species as well as higher concentrations of Mn4+, surface adsorbed oxygen species and active Mn–O bonds. Therefore, this catalyst possessed better reducibility and the best oxygen mobility, which were conducive to the catalytic oxidation of toluene. In addition, the DRIFT spectra results showed that more benzoic acid intermediate was deeply oxidized to carbonate species at a lower temperature on the surface of MNMA catalyst, which improved the low temperature activity for toluene oxidation.


 Please wait while we load your content...
Please wait while we load your content...