Tracking the role of trans-ligands in ruthenium–NO bond lability: computational insight†
Abstract
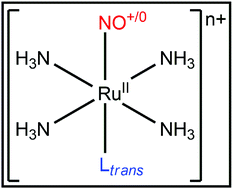
Nitric oxide (NO) is an important endogenously produced molecule. Ruthenium–NO tetraamine complexes appear as model structures to investigate the control of nitric oxide bioavailability. NO release typically occurs through the trans-[RuII(NH3)4Ltrans(NO+)]3+/2+ + e− → trans-[RuII(NH3)4Ltrans(NO0)]2+/+ reduction reaction, followed by the trans-[RuII(NH3)4Ltrans(NO0)]2+/+ + H2O → trans-[RuII(NH3)4Ltrans(H2O)]2+/+ + NO0 hydrolysis reaction. The choice of the NO trans ligand, Ltrans, is fundamental to control the Ru–NO bond stability. Here, the nature and charge influences of Ltrans were evaluated considering the following ligands Ltrans = σ-donors (NH3 and H−), π-donors (H2O and NH2−), and π-donors and π-acceptors (CO and CN−) through the ZORA-BP86/TZ2P computational model. Energy Decomposition Analysis in conjunction with the Natural Orbitals for Chemical Valence methodology (EDA-NOCV) showed that molecules with Ltrans = H2O and NH2− promoted larger Ru–NO stabilization than complexes with Ltrans = NH3 and H−, respectively, mainly due to the interaction orbital energy (ΔEoi). The opposite trend was observed in compounds with Ltrans = CO and CN− compared to structures with Ltrans = H2O and NH2−. The study of the charge distribution, performed using the Voronoi Deformation Density (VDD) method, and the topological analysis of the electron density, realized using the Quantum Theory of Atoms in Molecules (QTAIM), on the investigated complexes indicated that Ltrans with negative charge promoted, in general, predominantly a decrease of the electron flux in the Ltrans(σ) → Ru(dσ*) ← NO(σ) process.


 Please wait while we load your content...
Please wait while we load your content...