CK2 inhibition, lipophilicity and anticancer activity of new N1versus N2-substituted tetrabromobenzotriazole regioisomers†
Abstract
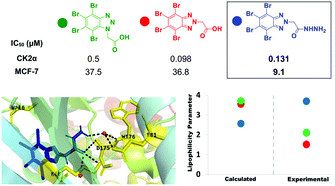
A new series of antiproliferative casein kinase 2α (CK2α) inhibitors were synthesized incorporating either a hydrophilic group (carboxylic or hydrazide) or a hydrophobic group (ester) at N1 or N2 of 4,5,6,7-tetrabromobenzotriazole (TBBt). New compounds were prepared via N-alkylation of TBBt followed by base-catalysed hydrolysis or hydrazinolysis. All the compounds demonstrated low sub-micromolar inhibition of CK2α and antiproliferative activity against both breast and lung cancer cell lines (MCF-7, and A549, respectively), at low micromolar concentrations with N2-regioisomers exhibiting higher activity than their corresponding N1-isomers. The most active compound incorporates an acetic acid hydrazide moiety at the N2 of the TBBt triazole nucleus with IC50 at 0.131 μM (CK2α), 9.1 μM (MCF-7) and 6.3 μM (A549). It induced apoptosis in the MCF-7 cell line through upregulation of bax (pro-apoptotic gene) four to five times higher than the corresponding ester or acid analogues. Molecular docking suggests that the hydrophilic group at N2 of the TBBt triazole nucleus provides binding with important residues (Asp175, Lys68 and Trp176) in the ATP binding site of the CK2α enzyme. We are the first to experimentally estimate the lipophilicity of TBBt derivatives. Our study demonstrated that TBBt hydrazides are more lipophilic than their corresponding acids in contrast to the contradicting calculated lipophilicity using four well-known software programs. This may explain the higher anticancer activity of the most active hydrazide over its corresponding acid despite their nearly equipotent enzyme inhibition.


 Please wait while we load your content...
Please wait while we load your content...