Design, synthesis, and anti-proliferative evaluation of 1H-1,2,3-triazole grafted tetrahydro-β-carboline-chalcone/ferrocenylchalcone conjugates in estrogen responsive and triple negative breast cancer cells†
Abstract
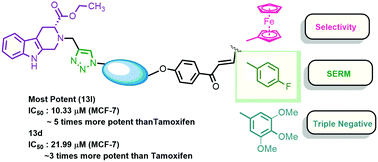
A series of 1H-1,2,3 triazole grafted tetrahydro-β-carboline-chalcone/ferrocenylchalcone conjugates were synthesized and in vitro evaluated against estrogen responsive (MCF-7) and triple negative (MDA-MB-231) breast cancer cells. Comparative analysis revealed the improvement of selectivity towards the estrogen responsive cells with the inclusion of a ferrocene core. The most potent compounds of the series were 13l (R = 4-F, n = 3), which exhibited an IC50 value of 10.33 μM against MCF-7 and was ∼5 fold more potent than the standard drug Tamoxifen, while 13d (R = 2,3,4-trimethoxy, n = 5) exhibited an IC50 value of 21.99 μM against the MDA-MB-231 cells, being ∼3 fold more potent than Tamoxifen. The experimental results were further supported by the molecular docking studies in the ligand binding domain of ERα and a greater binding affinity has been attributed to the energetically favourable fit and balance between the hydrophobic and hydrophilic interactions in the ERα active site.


 Please wait while we load your content...
Please wait while we load your content...