Imidazole diarylethene switches: an alternative to acid-gated photochromism†
Abstract
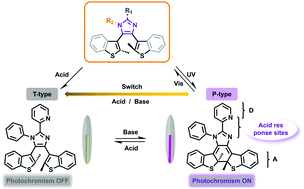
We prepared five diarylethenes containing 2-aryl-imidazole as the ethene bridge (L1–L5), and introduced response sites in imidazole rather than in the traditional appended aryl units to regulate their photochromism and thermal stability under acid stimulus. The results show that we can change the thermal stability from P- to T-type, and prevent their photoactivity by acidification, and it is clarified that the stronger the acid or the more acid added, the faster the decay rate of the diarylethene photostability. Furthermore, the prohibited photoactivity could be restored by neutralization with an equimolar amount of base.


 Please wait while we load your content...
Please wait while we load your content...