Synthesis and herbicidal application of turpentine derivative p-menthene type secondary amines as sustainable agrochemicals†
Abstract
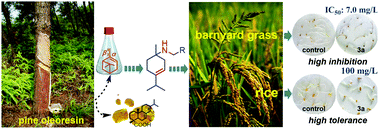
In this study, we have synthesized a series of novel turpentine derivative p-menthene type secondary amines (3a–o), which can be potentially used as sustainable crop protection agrochemicals. The synthesized compounds were analyzed by FTIR, 1H NMR and 13C NMR spectroscopy and HRMS. Their herbicidal activities against barnyard grass were evaluated by the classical culture dish method based on bioassays. Preliminary results indicated that turpentine derivative p-menthene type secondary amines exhibited herbicidal activity equivalent to or even higher than that of commercial herbicide diuron, among which the herbicidal activity of 3a, 3b, 3i and 3j against the root growth of barnyard grass was almost two times higher than that of glyphosate. Further preemergent application demonstrated that compound 3a enabled a strong and broad spectrum against some weeds at a dosage of 100 mg L−1. At a lower concentration of 10 mg L−1, compound 3a exhibited better preemergent herbicide activity than that of the commercial diuron. Moreover, compound 3a was selectively harmless to rice, wheat, cucumber and radish at a dosage of 100 mg L−1, while diuron was harmful to most of the tested crops at this high concentration. All of the above compounds are nontoxic to human and animal cells based on the cytotoxicity test, suggesting that sec-p-menth-3-enylamine derivatives can be potentially applied as botanical herbicides for weed control.


 Please wait while we load your content...
Please wait while we load your content...