Mild and efficient synthesis of trans-3-aryl-2-nitro-2,3-dihydrobenzofurans on water†
Abstract
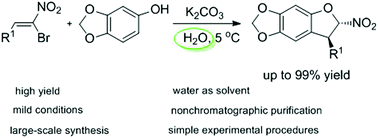
An environmental-friendly and mild method has been successfully developed for the synthesis of 3-aryl-2-nitro-2,3-dihydrobenzofurans through domino Friedel–Crafts/substitution reaction of (Z)-bromonitrostyrenes with sesamol. A variety of substrates performed well in this reaction, and the corresponding 3-aryl-2-nitro-2,3-dihydrobenzofurans were obtained in excellent yields (up to 99%). Significantly, water served as the sole green solvent. Remarkably, the crude products were purified by simple filtration, washing with water and drying in oven without any organic solvents or silica gel.


 Please wait while we load your content...
Please wait while we load your content...