Synthesis, crystal structure, and optical properties of fluorinated poly(pyrazole) ligands and in silico assessment of their affinity for volatile organic compounds†
Abstract
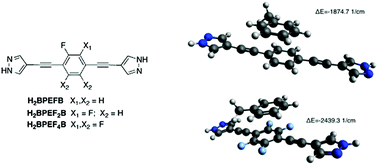
Three new fluorinated bis(pyrazoles), namely: 1,4-bis(1H-pyrazol-4-ylethynyl)-2-fluorobenzene (H2BPEFB), 1,4-bis(1H-pyrazol-4-ylethynyl)-2,3-difluorobenzene (H2BPEF2B) and 1,4-bis(1H-pyrazol-4-ylethynyl)-tetrafluorobenzene (H2BPEF4B), have been synthesized taking advantage of Sonogashira coupling reactions, and characterized as per their crystal and molecular structure, spectroscopic and dielectric properties, and hydrophobicity. In the crystal structures, the three molecules, whose deviation from planarity increases on increasing the fluorination degree, interact by means of hydrogen bonds, forming 2D supramolecular layers. Notably, the absorption and fluorescence emission properties are only slightly affected by the fluorination degree in both the solid state and solution. Furthermore, the spectral line-shapes are weakly dependent on the environment when dissolved in a number of solvents of different polarity and hydrogen-bonding affinity. On the other hand, the dielectric constant monotonically increases on increasing the number of fluorine atoms. In silico molecular modeling with time-dependent density functional theory has offered a valuable means to rationalize the above mentioned behaviors and has shed some light on the ligand affinity towards representative gases – H2O and CO2 – and organic solvents – toluene.


 Please wait while we load your content...
Please wait while we load your content...