Degradation of tetracycline in water using Fe3O4 nanospheres as Fenton-like catalysts: kinetics, mechanisms and pathways†
Abstract
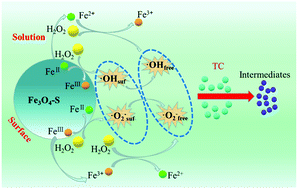
Fe3O4 nanospheres (Fe3O4-S) synthesized via a facile one-pot solvothermal method were used for H2O2 activation and tetracycline (TC) elimination from aqueous solutions. It can be found that more than 80% of TC was degraded in the Fe3O4-S/H2O2 system. Besides, batch experiments were conducted to investigate the effects of different parameters such as catalyst dosages, H2O2 concentration, pH values and temperature on the degradation of TC, and these experimental results were also described by the pseudo-first-order model. Radical quenching experiments and an electron paramagnetic resonance (EPR) technique revealed that ˙OH and ˙O2−/˙HO2 were involved and ˙OH generated on the surface of Fe3O4-S played a main role in TC degradation. The XPS observations demonstrated that the surface FeII participated in the H2O2 activation through the redox reactions. Moreover, thirteen intermediate products were monitored by the LC–MS and possible degradation pathways of TC were accordingly proposed. The Fe3O4-S catalyst exhibited good reusability and the catalytic performance of it did not show any significant decrease even after five trials. It was worth noting that the optimal pH for TC degradation was expanded to neutral pH conditions by using the Fe3O4-S/H2O2 system. Additionally, Fe3O4-S was easily separated from the reaction solutions by virtue of its magnetism (66.8 emu g−1), which is beneficial for reuse of the catalysts.


 Please wait while we load your content...
Please wait while we load your content...