Metal-free iodine-promoted direct synthesis of unsymmetrical triarylmethanes†
Abstract
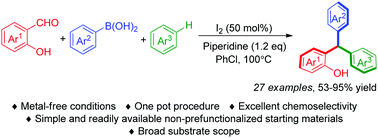
A highly efficient strategy to synthesize completely unsymmetrical triarylmethanes promoted by iodine under metal-free conditions has been successfully developed. Three different aryl groups were introduced into triarylmethanes in a one-pot reaction from inexpensive and readily available salicylaldehydes, arylboronic acids and arenes via o-QM intermediates generated in situ, delivering a wide range of unsymmetrical triarylmethanes bearing various functional groups in good yields with excellent chemoselectivity.


 Please wait while we load your content...
Please wait while we load your content...