Indium(iii) {2}-metallacryptates assembled from 2,6-dipicolinoyl-bis(N,N-diethylthiourea)†
Abstract
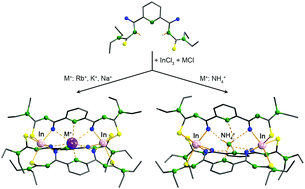
Reactions of 2,6-dipicolinoylbis(N,N-diethylthiourea), H2Lpy, InCl3, MCl salts (M = Rb+, K+, Na+ or NH4+) and NEt3 in methanol give cationic {2}-metallacryptates of the composition [M⊂{In2(Lpy)3}]+ (M+ = Rb+, K+, Na+ or NH4+). They can be isolated as PF6− salts. The structural characterization of the products by means of spectroscopic methods and X-ray crystallography reveals host–guest assemblies, in which a monovalent guest cation M+ is encapsulated in the cryptand-like host compound {In2(Lpy)3}. The host–guest directional interactions are coordination or hydrogen bonds depending on the nature of the guest cation. A 15N NMR spectroscopic study on the inclusion compound with 15NH4+ cations reveals a dynamic structure of the corresponding {2}-metallacryptate in solution and partial exchange of the ammonium ions by coordinating solvents.


 Please wait while we load your content...
Please wait while we load your content...