Support morphology-dependent catalytic activity of the Co/CeO2 catalyst for the aqueous-phase hydrogenation of phenol†
Abstract
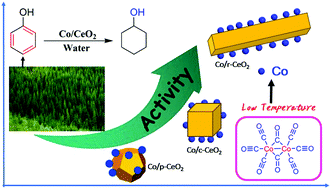
Herein, three Co/CeO2 catalysts with various support morphologies were prepared by Co2(CO)8 decomposition at a low temperature of 180 °C on the ceria plane such as CeO2 nanocubes (c-CeO2), nanorods (r-CeO2), and nanopolyhedrons (p-CeO2). The Co/r-CeO2 catalyst shows a much higher phenol conversion (82.5%) than Co/c-CeO2 (47.9%) and Co/p-CeO2 (24.7%) at 150 °C and 3 MPa H2 in water. We demonstrate that the less hydrophilic Co/r-CeO2 catalyst inhibits the adsorption of water and further promotes the adsorption of phenol. Moreover, the morphology effect and oxygen vacancies in different chemical environments of the support provide active sites for the dissociation and adsorption of phenol. The high concentration of oxygen vacancies exposed on the high active crystal plane leads to more efficient catalytic activity for the hydrogenation of phenol.


 Please wait while we load your content...
Please wait while we load your content...