Photodegradation of porphyrin-bound hIAPP(1–37) fibrils
Abstract
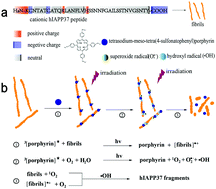
Amyloid deposits in pancreatic islets of type 2 diabetes mellitus (T2DM) are mainly comprised of human islet amyloid polypeptide (hIAPP), which is believed to be generated as a paracrine hormone along with insulin secretion. Misfolded hIAPP in islet beta cells is associated with T2DM. To reduce the risk of T2DM, amyloid aggregate degradation to remove insoluble amyloid deposits has been proven to be a preventive strategy against T2DM. However, the development of effective approaches and degradation mechanisms needs to be further addressed. Herein, we introduce tetrasodium-meso-tetra(4-sulfonatophenyl)porphyrin binding to hIAPP37 aggregates by electrostatic interactions, which could enhance the degradability of hIAPP37 fibrils under photo-irradiation. The morphology change of hIAPP fibrils could be easily revealed through the effect of photoexcited porphyrin without any additives under neutral conditions. The photoexcited porphyrin-induced degradation of amyloid fibrils may be attributed to reactive oxygen species destabilizing the β-sheet secondary structure under photo-irradiation. Moreover, the degraded products of hIAPP37 fibrils show low cytotoxicity to INS cells. This work could be beneficial for the molecular design of novel photodegradation agents of amyloid fibrils and also expand the biomedical application of porphyrin for photosensitization.


 Please wait while we load your content...
Please wait while we load your content...