Pteridine derivatives: novel low-molecular-weight organogelators and their piezofluorochromism†
Abstract
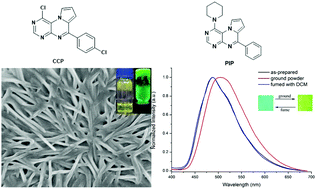
Here, π-conjugated compounds based on pteridine derivatives were synthesized and their self-assembling behaviors in a variety of organic solvents were studied. Although there were no conventional gelation groups, such as alkyl groups, amide bonds, or hydrogen bond units, the synthesized pteridines substituted with phenyl, p-chlorophenyl and p-fluorophenyl exhibited different self-assembling abilities, and the p-chlorophenyl-substituted pteridine derivative CCP showed excellent gelation ability with a minimum gelation concentration (MGC) of 2 mg mL−1 in isopropanol, n-butanol and amylene alcohol. In addition, the fluorescence response to mechanical forces for the 4-amino- or hydroxyl-substituted pteridine derivatives, namely, PYP, PIP, DBP, BNP and OHP was obtained. Interestingly, the pyrrolidine- and dibutyl amino-substituted compounds PYP and DBP showed a blue-shift in emission after grinding, while the piperidine-, benzyl amino- and hydroxyl-substituted compounds PIP, BNP and OHP showed a red-shift in emission after grinding. The single-crystal structures of PYP and PIP excluded that the distance corresponding to the π–π interactions led to the differences in MFC properties.


 Please wait while we load your content...
Please wait while we load your content...