Strong metal–support interactions between palladium nanoclusters and hematite toward enhanced acetylene dicarbonylation at low temperature†
Abstract
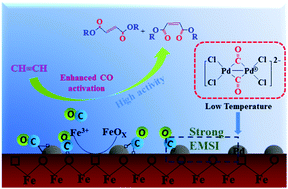
Dicarbonylation is an atom-efficient and straightforward method for the direct incorporation of two carbonyl functionalities into organic molecules in a wide range of homogeneous catalytic processes. Preparation of analogously performing heterogeneous supported catalysts is of great importance. Here, a four-fold increase in the acetylene dicarbonylation activity of a palladium-based heterogeneous catalyst was obtained. The strong metal–support interaction (SMSI) between Pd nanoclusters and α-Fe2O3 support was responsible for enhancing the low temperature catalytic activity, which was confirmed by X-ray photoelectron spectroscopy (XPS) and H2 temperature program reduction (H2-TPR) analyses. The modified electronic properties of high-valent Pdδ+ clusters could lower the CO chemisorption barrier energy. In situ diffuse reflectance infrared Fourier transform spectroscopy (in situ DRIFTS) was performed to confirm that the interface provided more CO chemisorption and (co-)activation sites. The acetylene dicarbonylation over Pd/α-Fe2O3 proceeds with a low apparent activation energy (36.26 kJ mol−1), which accounted for the high activity toward acetylene dicarbonylation at low temperature. Our study unravels that using a transition metal oxide as a support is a promising strategy for enhancing the efficiency of dicarbonylation and using earth-abundant catalytic materials can reduce the catalyst cost.


 Please wait while we load your content...
Please wait while we load your content...