Colorimetric detection of Cs+ based on the nonmorphological transition mechanism of gold nanoparticles in the presence of Prussian blue†
Abstract
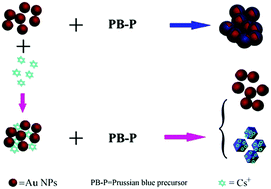
With the increasing market demand of cesium, the development of rapid, accurate, and on-site detection methods for cesium ions in saline lake brine has important significance on the development and comprehensive utilization of resources in saline lakes. In addition, radioactive cesium has great and long-term harm to human beings, and it is of great significance for the prompt detection of radioactive Cs+ ions. In this work, a new strategy for the colorimetric detection of Cs+ using Au NPs and Prussian blue (PB) based on a nonmorphological transition mechanism was proposed. When a PB precursor solution was added into a Au NP solution, Fe3+ was reduced to Fe2+ by the citric acid in the Au NP solution, while the PB coated the surface of the Au NPs, which will cause the change in color and surface plasmon resonance absorption of the Au NPs. On the other hand, when the PB precursor solution was added into a Au NPs solution with Cs+ ions, there was a competition between K+ and Cs+, and more stable complexes were formed due to the strong affinity between Cs+ and PB. At this time, the Au NP solution remained wine red. The good linear relationship (R2 = 0.9933) indicates that Au NPs and Prussian blue-based colorimetric probes can be used for the qualitative and quantitative determination of Cs+. The naked eye detection limit was 30 μM, and the UV-vis detection limit was 19 μM.


 Please wait while we load your content...
Please wait while we load your content...