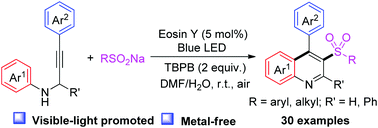
Synthesis of 3-sulfonylquinolines by visible-light promoted metal-free cascade cycloaddition involving N-propargylanilines and sodium sulfinates†
Abstract
A visible-light promoted radical cascade reaction of N-propargylanilines with sodium sulfinates as sulfonyl radical precursors was developed under metal-free conditions. This mild protocol represents a speedy and efficient method for synthesizing 3-sulfonylquinolines via the formation of C–S and C–C bonds in one step.


 Please wait while we load your content...
Please wait while we load your content...