Indium and thallium extraction into betainium bis(trifluoromethylsulfonyl)imide ionic liquid from aqueous hydrochloric acid media
Abstract
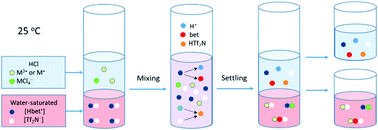
The partitioning behavior of indium and thallium ions between aqueous hydrochloric acid solutions and hydrophobic betainium bis(trifluoromethylsulfonyl)imide ionic liquid was studied using a standard liquid–liquid extraction technique. The experiments were mainly carried out by means of a radiochemical method, using 111In and 201Tl medical radionuclides at an ultra-trace concentration. Both mono- and trivalent thallium were utilized and the highest oxidation state (3+) was produced by adding either chlorine or bromine water to the aqueous phase prior to extraction. The metal ion transfer was optimized by varying the aqueous concentration of hydrochloric acid and by introducing zwitterionic betaine as an extracting agent into the aqueous phase. It was found that the highest D values of In(III), Tl(III) and Tl(I) in the investigated chemical systems were up to 200, 70, and 1, respectively. The influence of the addition of lithium salt, having common with the ionic liquid counterion, and the variation of mixing time on the distribution ratio of the metal ions were also studied. To understand the underlying extraction mechanism of the metal ions and to propose the predominant extracted species, mathematical models based on ion pair formation and ion exchange were developed.


 Please wait while we load your content...
Please wait while we load your content...
