Enhanced photocatalytic degradation of 2-thiobenzimidazole by the tris(8-quinolinolato)cobalt(iii) complex through peroxide adduct formation: theoretical and experimental investigations†
Abstract
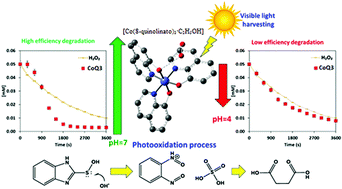
Photo-initiated oxidative degradation of 2-thiobenzimidazole (2-TBI) was studied using different semiconducting metal complexes derived from 8-hydroxyquinoline with the general formula, [MQ3C2H5OH] [Q = 8-quinolinolato anion; M = Cr3+, (CrQ3); Co3+, (CoQ3); Fe3+, (FeQ3)]. The degradation kinetics was monitored via UV-vis spectroscopy perceiving the changes in absorbance to subtract. The effect of pH and visible light irradiation on degradation was studied observing a significant increment in the oxidation rate with the presence of visible light whenever CoQ3 is used as a catalyst. By using density functional theory (DFT), it was found that the transition state energy of the adduct formed between CoQ3 and H2O2 is very low compared with other studied catalysts, which activates the formation of ˙OH radicals from H2O2 increasing the 2-TBI degradation rate. In addition, a thermodynamically feasible degradation mechanism was proposed by calculating energy barriers involved in the 2-TBI oxidation pathway.


 Please wait while we load your content...
Please wait while we load your content...