Solvent-dependent regioselectivity of 2′-deoxyadenosine alkylation by 9-aminomethyl derivatives of SN38†
Abstract
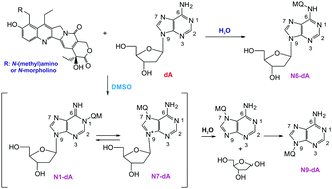
The mechanism of alkylation of the 2′-deoxyadenosine (dA) by potential topoisomerase I (Top I) inhibitors, from the camptothecin family, is described in water and in DMSO-d6. The products of the reactions depend on the solvent; however in both cases the thermodynamic products are formed. The investigated compounds in DMSO solution unexpectedly alkylate the N9 nitrogen atom of 2′-deoxyadenosine, preceded by an irreversible deglycosylation process, whereas the N6 nitrogen atom is alkylated in buffered water solution. The products of the reaction have been confirmed by NMR and MS techniques. This is the first evidence of spontaneous alkylation of 2′-deoxyadenosine by compounds from the camptothecin family, which are biologically active in vitro against several cancer cells.


 Please wait while we load your content...
Please wait while we load your content...