Synthesis and characterization of a magnetic graphene oxide/Zn–Ni–Fe layered double hydroxide nanocomposite: an efficient mesoporous catalyst for the green preparation of biscoumarins†
Abstract
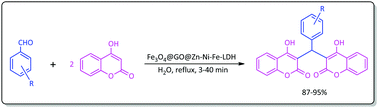
In this study, a nanocomposite of Fe3O4@GO@Zn–Ni–Fe-layered double hydroxide was synthesized as a novel and efficient mesoporous magnetic nanocatalyst. The synthesis was carried out via the immobilization of Fe3O4 MNPs (magnetic nanoparticles) on graphene oxide and then the layering of Zn–Ni–Fe-layered double hydroxide moieties on the constituents of Fe3O4@GO. The magnetic graphene oxide/layered double hydroxide system was then characterized using SEM, EDX, FTIR, XRD, TEM and VSM analyses. The catalytic activity of the prepared composite system was further studied towards one-pot Knoevenagel–Michael reaction of aromatic aldehydes with 4-hydroxycoumarin in water. All reactions were carried out under reflux conditions giving biscoumarin materials in 87–95% yields within 3–40 min. This method has noteworthy advantages in terms of mild reaction conditions, short reaction times, utilizing water as an environmental friendly solvent and applying a magnetically separable catalyst system.


 Please wait while we load your content...
Please wait while we load your content...