Combined experimental, DFT theoretical calculations and biological activity of sulfaclozine azo dye with 1-hydroxy-2-naphthoic acid and its complexes with some metal ions†
Abstract
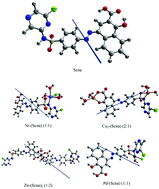
Sulfaclozine-1-hydroxy-2-naphthoic acid azo dye (Scna) and its metal complexes with Ni(II) Cu(II), Zn(II) and Pd(II) metal ions have been prepared and characterized using different techniques. The non-isothermal degradation of Pd(II)–(Scna) was studied using Flynn–Wall–Ozawa and Starink methods at various heating rates (5, 10, 15 and 20 °C min−1) from the thermogravimetry/derivative thermogravimetry (TG/DrTG) and differential thermal analysis (DTA) curves. The values of the activation energy (E) obtained using the two methods are in impeccable harmony. Theoretical studies (density functional theory, DFT) were also carried out to support the corresponding experimental results. Computational calculations were achieved using a DFT/GEN level of theory. Theoretical aspects, in terms of geometrical optimization and molecular charge density plots are also mentioned using the standard basis set LANL2DZ for metal ions. A suitable distorted square planar for Pd–(Scna), distorted tetrahedral for Cu2–(Scna), and distorted octahedral with Ni–(Scna) and Zn–(Scna)2 structures were observed for the complexes. The electronic structure and nonlinear optical parameters (NLO) of the complexes were calculated. The nature of the interaction between the metal ions and the ligand, molecular stability and bond strengths have been studied using DFT calculations employing natural bond orbital (NBO) analysis. The complexes are non-planar and demonstrate the expected optical properties. The synthesized ligand (Scna) and its complexes were screened for antimicrobial activity against bacteria, Staphylococcus aureus as the Gram-positive and Escherichia coli as the Gram-negative, using the disc diffusion method. The Zn–(Scna)2 complex displayed the highest activity against both bacteria.


 Please wait while we load your content...
Please wait while we load your content...