An efficient ruthenium(ii) tris(bipyridyl)-based chemosensor for the specific detection of cysteine and its luminescence imaging in living zebrafish†
Abstract
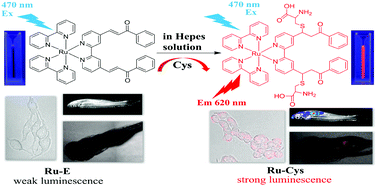
A novel luminescent chemosensor based on an α,β-unsaturated ketone conjugated Ru(bpy)32+ derivative (Ru-E) has been designed and synthesized for the highly selective and sensitive detection of cysteine in 100% aqueous solutions. Owing to the conjugate 1,4-addition of cysteine to α,β-unsaturated ketones, the non-luminescent chemosensor Ru-E could specifically react with cysteine to form the corresponding product, Ru-Cys, accompanied by remarkable luminescence enhancement at 620 nm. Job's plot and mass spectrometry analysis indicate a 1 : 2 binding stoichiometry between Ru-E and Cys. The sensing mechanism is confirmed by NMR and mass and emission spectrometry. Moreover, Ru-E exhibited very low cytotoxicity and was successfully applied to image endogenous thiols both in living bone mesenchymal stem cells (BMSCs) and zebrafish. In addition, Ru-E showed biological application for monitoring thiols in newborn-calf serum. The results of this work demonstrate that Ru-E would be a useful tool for physiological and pathological studies involving biothiols.


 Please wait while we load your content...
Please wait while we load your content...