Electrocatalytic reduction of protons to hydrogen by a copper complex of the pentadentate ligand Dmphen-DPA in a nonaqueous electrolyte†
Abstract
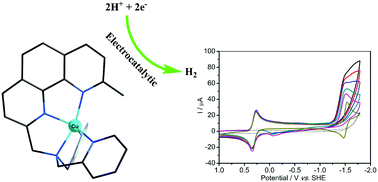
A copper complex based on the pentadentate aminopyridine ligand [(9-methyl-1,10-phenanthrolin-2-yl)methyl]bis-(pyridin-2-ylmethyl)amine (Dmphen-DPA), namely [Cu(Dmphen-DPA)](ClO4)2 (1), was synthesized and characterized by elemental analysis, HR-MS spectroscopy and X-ray single crystal diffraction. The complex has five-coordinated solid-state structures, but has an open coordination site in acetonitrile. The electrochemical studies reveal that complex 1 has an electrocatalytic proton reduction activity in acetonitrile, when using acetic acid as a proton source with icat/ip ∼ 2.5. DFT calculations suggest that the parent copper complex undergoes two successive reductions to generate the radical species [Cu(I)(Dmphen-DPA˙−)]0, which is protonated at the low-valence copper center to afford a reactive CuII–H intermediate. The CuII–H species combines bimolecularly and generates H2via reductive elimination.


 Please wait while we load your content...
Please wait while we load your content...