A novel and ultrasensitive yellow to taupe brown colorimetric sensing and removal method for Hg(ii) based on the thermosensitive poly(N-isopropyl acrylamide) stabilized silver nanoparticles†
Abstract
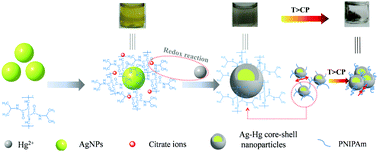
Many colorimetric sensors based on noble metal nanoparticles for the detection of mercury ion (Hg2+) have attracted widespread interest from academia in recent years. A critical issue is how to realize the detection of Hg2+ with high sensitivity and selectivity. A novel colorimetric sensor based on AgNPs stabilized by linear thermoresponsive poly(N-isopropyl acrylamide) (PNIPAm) was developed for the application of detecting Hg2+ with a color change from yellow to taupe brown due to the formation of Ag–Hg core–shell nanoparticles. The AgNP/PNIPAm sensor exhibited good selectivity and high sensitivity (LOD = 75 nM) with a good linear relationship not only in the ultrapure water (R2 = 0.9903) but also in the tap water (R2 = 0.9905). More importantly, based on the thermosensitivity of PNIPAm, this sensor could also achieve the separation of Ag–Hg composites with enriching efficiencies of 97.60% and 95.40% for Ag and Hg, respectively, which can avoid serious secondary pollution. Therefore, this dual-functional nanocomposite has important potential implications in the detection and separation of Hg2+.


 Please wait while we load your content...
Please wait while we load your content...