An integrative process of the simultaneous catalytic oxidation of NO, Hg0 and toluene from sintering flue gas by the natural ferrous manganese ore
Abstract
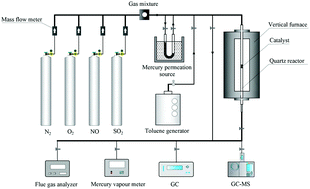
NOx, Hg0 and VOCs are the major atmospheric pollutants produced in the sintering process, which cause serious harm to human health and the ecological environment. In this study, the simultaneous selective catalytic oxidation (SCO) of NO, Hg0 and toluene from sintering flue gas by the natural ferrous manganese ore was investigated in a fixed bed reactor. Moreover, the natural ferrous manganese ore was characterized by BET, XRD, XPS, H2-TPR, O2-TPD and in situ DRIFTS. The results indicate that Mn4+ and Fe3+ in the natural ferrous manganese ore are the main active components, which result in higher oxidation activity of the natural ferrous manganese ore. The maximum efficiencies of NO, Hg0 and toluene oxidation over the natural ferrous manganese ore are 95.29%, 83.0% and 81.9%, respectively, at 300 °C under the GHSV of 18 000 h−1; in addition, CO, benzaldehyde and benzoic acid are the main byproducts produced from toluene oxidation over the natural ferrous manganese ore, whose concentration first increases and then decreases as the temperature increases from 50 to 300 °C. The co-existence of H2O and SO2 dramatically reduces the oxidation activity of the natural ferrous manganese ore.


 Please wait while we load your content...
Please wait while we load your content...